
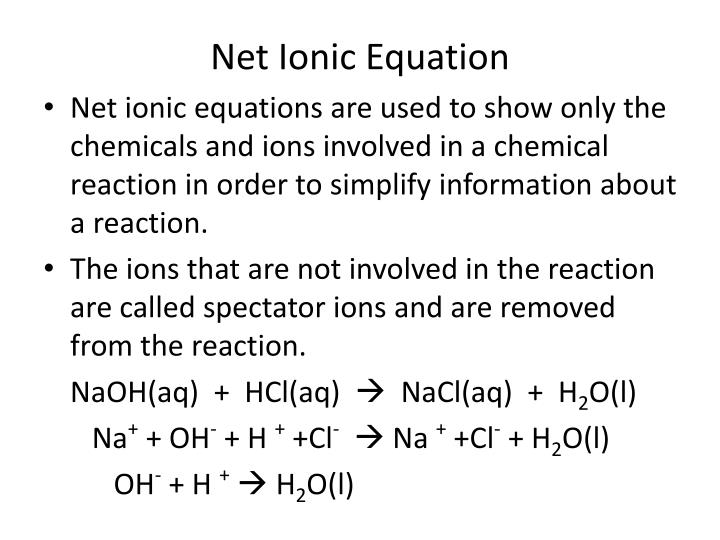
In the process, the chlorine is reduced to chloride ions. In reality, you almost always start from the electron-half-equations and use them to build the ionic equation.Įxample 1: The reaction between chlorine and iron(II) ionsĬhlorine gas oxidises iron(II) ions to iron(III) ions. That's doing everything entirely the wrong way round! In the example above, we've got at the electron-half-equations by starting from the ionic equation and extracting the individual half-reactions from it. Working out electron-half-equations and using them to build ionic equations Note: If you aren't happy about redox reactions in terms of electron transfer, you MUST read the introductory page on redox reactions before you go on. These two equations are described as "electron-half-equations" or "half-equations" or "ionic-half-equations" or "half-reactions" - lots of variations all meaning exactly the same thing!Īny redox reaction is made up of two half-reactions: in one of them electrons are being lost (an oxidation process) and in the other one those electrons are being gained (a reduction process). This shows clearly that the magnesium has lost two electrons, and the copper(II) ions have gained them. You can split the ionic equation into two parts, and look at it from the point of view of the magnesium and of the copper(II) ions separately. There are links on the syllabuses page for students studying for UK-based exams. You should be able to get these from your examiners' website. How do you know whether your examiners will want you to include them? The best way is to look at their mark schemes. Practice getting the equations right, and then add the state symbols in afterwards if your examiners are likely to want them. This topic is awkward enough anyway without having to worry about state symbols as well as everything else. Note: I am going to leave out state symbols in all the equations on this page. When magnesium reduces hot copper(II) oxide to copper, the ionic equation for the reaction is:

Take your time and practise as much as you can.
It is a fairly slow process even with experience.

This is an important skill in inorganic chemistry.ĭon't worry if it seems to take you a long time in the early stages.
Ionic equation how to#
This page explains how to work out electron-half-reactions for oxidation and reduction processes, and then how to combine them to give the overall ionic equation for a redox reaction. WRITING IONIC EQUATIONS FOR REDOX REACTIONS Writing ionic equations for redox reactions


 0 kommentar(er)
0 kommentar(er)
